Brandeis Researchers Take a New Look at ALS
Jeff Agar
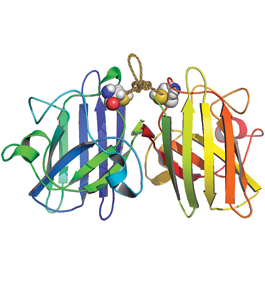
Depiction of the chemical rope that stabilizes mutant protein responsible for familial ALS.
by Laura Gardner
In the constellation of neurodegenerative diseases being researched in laboratories around the world, amyotrophic lateral sclerosis (ALS), commonly known as Lou Gehrig’s disease, is surely one of the most devastating. In its familial and sporadic forms not only is it always fatal, it strikes typically in middle age or earlier, eventually destroying most motor neurons throughout the body. Progressive muscle weakness and atrophy ensue, with death usually occurring in three to five years.
Recently, a team of Brandeis scientists published in the Proceedings of the National Academies of Science an innovative approach to treating the most common form of familial ALS. In the study, chemist Jeff Agar and his colleagues researched mutations in the gene that makes a particular protein, known as SOD1, responsible for causing much of the familial form of ALS.
Genetic mutations make the SOD1 protein unstable, causing it to fall apart into two identical pieces called monomers, which are sticky and prone to clumping up inside the axon, the long projection of the motor neuron that conducts electrical impulses. Motor neurons are a meter long; when the axon inside the neuron gets clogged, it eventually dies.
“Picture a tennis ball stuck to a small piece of double-sided tape. Now picture another. Turn the balls until both pieces of tape come into contact and that’s what scientists call a dimer, and it’s stable,” explains Agar. “It won’t stick to anything else. That’s what normal SOD1 looks like, and there are billions of SOD1 dimers in every motor neuron.”
SOD1 is one of the body’s hardest working antioxidants, and its job is to turn a dangerous free radical called superoxide into water.
“Now pull the tennis balls apart, turn one 180 degrees, stick them back together and there’s a sticky end. That’s what ALS-associated SOD1 mutants do. You could glue millions of these balls together if you had them, and a neuron has billions of them. What we’re trying to do is prevent this from happening,” says Agar.
Agar, along with postdoctoral fellow Jared Auclair and biochemists Greg Petsko and Dagmar Ringe, developed an ingenious “chemical rope” to tie the two monomers together, creating a stable dimer. Some ALS mutations stop SOD1 from doing its job, and the chemical ropes are even able to reactivate these SOD1 mutants and get them working again.
Now the scientists are creating a version of their proof-of-concept “chemical rope” that is potentially amenable to development into a drug. “This is only the beginning,” says Agar. “We have a lot more work to do before this could benefit ALS patients.”
While the familial form of Lou Gehrig’s affects only about 2 percent of all ALS cases, the researchers believe that perhaps 30 to 40 percent of cases where there is no genetic cause could potentially also benefit from the same treatment.
Recently, a team of Brandeis scientists published in the Proceedings of the National Academies of Science an innovative approach to treating the most common form of familial ALS. In the study, chemist Jeff Agar and his colleagues researched mutations in the gene that makes a particular protein, known as SOD1, responsible for causing much of the familial form of ALS.
Genetic mutations make the SOD1 protein unstable, causing it to fall apart into two identical pieces called monomers, which are sticky and prone to clumping up inside the axon, the long projection of the motor neuron that conducts electrical impulses. Motor neurons are a meter long; when the axon inside the neuron gets clogged, it eventually dies.
“Picture a tennis ball stuck to a small piece of double-sided tape. Now picture another. Turn the balls until both pieces of tape come into contact and that’s what scientists call a dimer, and it’s stable,” explains Agar. “It won’t stick to anything else. That’s what normal SOD1 looks like, and there are billions of SOD1 dimers in every motor neuron.”
SOD1 is one of the body’s hardest working antioxidants, and its job is to turn a dangerous free radical called superoxide into water.
“Now pull the tennis balls apart, turn one 180 degrees, stick them back together and there’s a sticky end. That’s what ALS-associated SOD1 mutants do. You could glue millions of these balls together if you had them, and a neuron has billions of them. What we’re trying to do is prevent this from happening,” says Agar.
Agar, along with postdoctoral fellow Jared Auclair and biochemists Greg Petsko and Dagmar Ringe, developed an ingenious “chemical rope” to tie the two monomers together, creating a stable dimer. Some ALS mutations stop SOD1 from doing its job, and the chemical ropes are even able to reactivate these SOD1 mutants and get them working again.
Now the scientists are creating a version of their proof-of-concept “chemical rope” that is potentially amenable to development into a drug. “This is only the beginning,” says Agar. “We have a lot more work to do before this could benefit ALS patients.”
While the familial form of Lou Gehrig’s affects only about 2 percent of all ALS cases, the researchers believe that perhaps 30 to 40 percent of cases where there is no genetic cause could potentially also benefit from the same treatment.
