Kern Lab Ups the Ante on Fighting Antibiotic Resistance
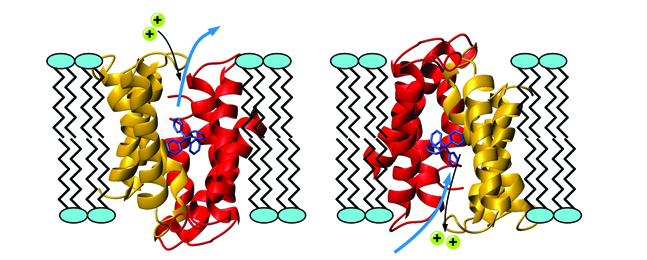
The protein EmrE, shown in red and gold, transports antibiotics across cell membranes, enabling disease bacteria to replicate and survive within the cell. The antibiotic, in blue, is being pulled outside the cell and exchanged with two protons, shown in turquoise. Courtesy of Michael Clarkson and the Protein Data Bank.
Here’s a sobering fact: Bacterial resistance to antibiotic treatment contributes to an estimated 99,000 deaths from hospital-acquired infections in the United States every year, according to the Centers for Disease Control and Prevention. Scientists have warned for years about the creeping epidemic of antibiotic resistance, making successful treatment of bacterial infections, from earaches to tuberculosis, a formidable public-health challenge.
One culprit in this epidemic is a drug-resistant protein called EmrE, one of a dozen or so such villains. A molecular kidnapper, it steals into cells where antibiotics are trying to stamp out infection and transports the antibiotics out of the cells, allowing the disease bacteria within the cell to mutate and evade destruction.
“These bacteria survive and duplicate every 20 minutes,” explains Dorothee Kern, a biochemist and Howard Hughes Medical Institute Investigator who is researching EmrE. “So the drug designed to kill that strain of bacteria no longer works. If you look at the statistics, the numbers of resistant strains of bacteria are growing exponentially.” In effect, the more antibiotics are used, the more new strains of bacteria evolve that are resistant to current antibiotics.
Using nuclear magnetic resonance spectroscopy, Kern studies how EmrE moves in real time to pump each antibiotic molecule out of the cell and then returns to grab another one. In a recent Nature paper, Kern and her colleagues, in particular a former postdoc, Katie Henzler-Wildman, who led the project, reported the shapes that the EmrE protein takes on while kidnapping antibiotics. This research is the first step in the arduous process of developing a drug treatment that could inhibit the EmrE protein from thwarting an antibiotic’s job in the cell. The challenge is to make a drug that kills the specific target, but nothing else.
Although a drug to fight antibiotic resistance may be years away, individuals can take steps now to slow the growth of antibiotic resistance. For instance, Kern cautions against purchasing antibacterial products like cutting boards, soaps and hand sanitizers because they contribute to bacterial mutations. “If you have an infection and use a high concentration of antibiotics, the bacteria are killed before they can adapt and change,” explains Kern. “If the dose is very small — as is the case with antibacterial products — bacteria may not all be killed, giving them time to mutate.”
