Wild and weird world of fluoride channels
Brandeis researchers discover how microbes survive the ubiquitous toxic ion
 Photo/istockphoto
Photo/istockphotoE. coli bacteria are protected from toxic fluoride by channel proteins
It’s not just in toothpaste and mouthwash — fluoride is found in just about everything from rocks and water to the soil and the sea. It is the 13th most abundant element in the Earth’s crust, and it’s extremely toxic to single-celled organisms such as bacteria and yeasts.
Yet these organisms survive and even flourish in fluoride-rich environments. How?
Brandeis University scientists have an answer that may have implications for the treatment of bacterial diseases such as tuberculosis.
In a paper published in August in the journal eLife, professor of biochemistry Christopher Miller reports microorganisms have evolved an unusual fluoride-specific ion channel to export toxic fluoride from the cell. Postdoctoral researchers Randy Stockbridge, Janice Robertson and Ludmila Kolmakova-Partensky contributed to the paper.
Ions are electrically charged molecules that need help from one of two types of membrane transport proteins — carriers and channels — to cross cell membranes. Carriers act like taxis, ferrying ions into and out of the cell. Channel proteins are more like one-way tunnels, forming a pore in the cell membrane that transports ions from areas of high concentration to areas of low concentration.
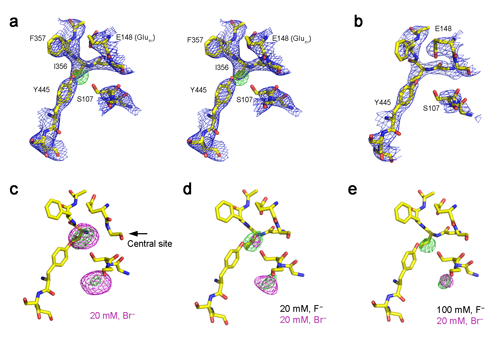 |
|
Fluoride (F-), highlighted in green, in the binding site of an exporter |
Fluoride ions are sneaky. They seep into cells in protonated form — as hydrofluoric acid — and when the acid breaks down in the cell’s higher pH, only the fluoride ions remain. Since hydrofluoric acid is easily able to permeate cell membranes, cells accumulate fluoride at higher levels than their extracellular environments.
That accumulation activates a family of transport proteins, called Fluc, to build a channel so specific that it can distinguish between the harmful fluoride and the closely related but harmless chloride, making it the most selective channel yet discovered. The channel is also unusual in that its structure is upside down and backwards from every other channel protein studied.
“This newly discovered ion channel violates all the rules of known channels,” says Miller, who is also an investigator with the Howard Hughes Medical Institute.
In examining how this channel works, Miller and his team disabled a microbe’s internal security system by using a bacterial strain whose Fluc gene is knocked out. When these bugs are exposed to fluoride, the ion accumulates and inhibits the production of energy by glycolysis and the synthesis of DNA and RNA. Miller and his team could then replace the Fluc gene, or similar genes from other organisms, to rescue the bacteria from this hypersensitivity to fluoride.
Since fluoride is mostly non-toxic to humans (but don’t eat your toothpaste), this strategy may be one avenue to explore in developing treatments for harmful bacterial diseases, Miller says. But for the moment, the channel’s weird, wild structure is captivating enough.
Categories: Research, Science and Technology





