Researchers make strides in understanding ALS
Petsko/Ringe laboratory successfully reverses toxicity of a mutated protein
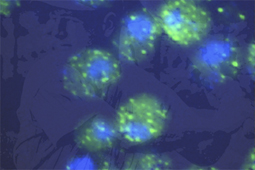 Photo/Gregory A. Petsko
Photo/Gregory A. PetskoYeast cells expressing human FUS/TLS (green spots) in cytosol, with blue stain of nucleus.
Brandeis researchers have made a significant advance in the effort to understand amyotrophic lateral sclerosis (ALS) by successfully reversing the toxicity of a mutated protein in the familial type of the disease.
Currently there is no cure or prevention for the disease, which affects nerve cells in the brain and the spinal cord. Most frequently referred to as Lou Gehrig’s disease, after its most famous victim, ALS typically causes death due to respiratory paralysis within three to five years of onset. The only approved drug, Riluzole, can extend the lifespan of some patients by three months.
In a paper published Tuesday, April 26 in PLoS Biology, the Petsko/Ringe laboratory reports success in blocking the lethal effects of the gene by placing several human genes into a yeast cell that shows many similar features to the disease-causing proteins.
Genes have been identified for many of the 10 percent of ALS cases that run in families. People with one of those mutant genes are likely to develop the disease. While a few of those genes might also contain mutations that increase risk for the more common forms of ALS, it’s one of those genes, FUS/TLS, which got the attention of the Petsko/Ringe team.
 |
| Shulin Ju, post-doctoral researcher and first author. |
Here is some of the biology and chemistry behind the research:
Post-mortem examinations of certain ALS victims show that the dying neurons contain clumps of the FUS/TLS protein. What’s interesting, says Gregory A. Petsko, professor of chemistry and biochemistry, is where these inclusions are.
“Normally this protein lives in the nucleus of the cell, which is where the chromosomes are,” says Petsko. “In this disease, it seems to move from the nucleus out into the cytoplasm of the cell, the main part, and that’s where it forms the inclusions that are associated with the disease.”
Petsko and Ringe’s team wanted to study this process in an organism on which they could perform sophisticated genetic screenings and detailed biochemical experiments, which can not be done in human cells. So they chose yeast.
“It may seem kind of crazy to think of doing yeast experiments on a human neurologic disease, since yeast has no brain or spinal cord or any neurons at all,” says Petsko, “But a yeast cell isn’t that different from a typical human cell.”
The team inserted the FUS/TLS gene into a yeast cell with the hope that it would create the same observable characteristics as the mutant protein does in a human cell. When they did, Petsko says, two remarkable things happened.
“First thing is that the human protein wasn’t in the nucleus, it moved to the cytoplasm of the cell just like it did in the human disease— and it formed inclusions,” says Petsko. “The second thing is that it killed the yeast cell, so we got in yeast a pretty faithful replication of some of the features of the human disease caused by mutation of this gene.”
 |
| Gregory A. Petsko, professor of biochemistry. |
The next step was to find out what part of the protein was necessary in order to keep it in the nucleus and what part was necessary to send it to the cytoplasm.
Petsko then asked, “If we started deleting sections of the protein could we force the protein to always be in the cytoplasm or always be in the nucleus?”
When they performed the experiment with yeast they found that the area of the gene where the disease-causing mutations occur was the area responsible for keeping it in the nucleus; when that area is mutated, the gene leaves the nucleus for the cytoplasm.
“We want to keep it in the nucleus but you can’t do that with the mutants easily because the part responsible for keeping it in the nucleus has been destroyed by the mutation, which is why you have the disease,” says Petsko.
They then asked whether they could prevent the yeast cells from being killed by this protein by placing some other protein inside.
In other words, Petsko says, could they find a protein that would rescue the cell from the toxicity of FUS/TLS?
By a series of genetic experiments described in the paper, they were able to identify several human genes which, when inserted along with FUS/TLS gene, rendered FUS/TLS protein no longer toxic to yeast. The cells survived.
“And then we got the surprise of our life,” says Petsko. “When we looked at those cells, FUS/TLS protein was still in the cytoplasm, and still forming inclusions. In other words, we were able to eliminate the toxicity of the protein without sending it back to the nucleus.”
What this told them was that aggregating and being in the cytoplasm didn’t necessarily have to be toxic as long as the rescue protein that they found was introduced.
That, says Petsko, got them really excited, because “if you can do that with the expression with another human gene you could probably do that with a drug.”
Categories: Research, Science and Technology





