SciFest showcases success in the sciences
This year's annual event featured prestigious alumni offering career tips and inspiration and students showcasing their research results.
 Heratch Ekmekjian
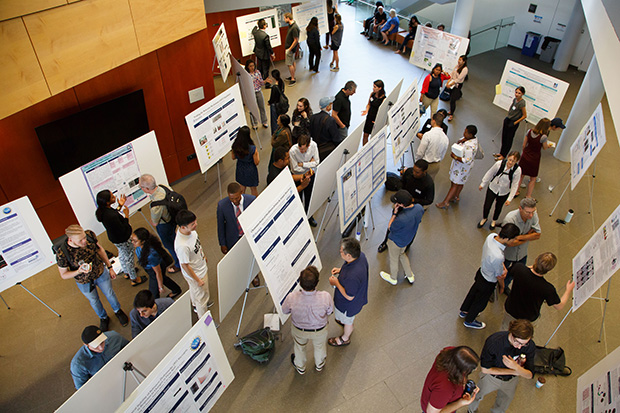
Heratch EkmekjianUndergraduates presented their posters to faculty, students and visitors at SciFest.
The students came to present the findings of their scientific research. The alumni, top scientists in their fields, came to inspire.
This year’s SciFest Summer Symposium and Poster Session highlighted the achievements of Brandeis undergraduates, the lasting impact of their bonds with professors, and the dedication and passion necessary to pursue a career in the sciences.
The day-long event was split into two parts: talks by four alumni in the Shapiro Campus Center followed by poster sessions in the Shapiro Science Center presented by 123 students. It was the ninth consecutive summer Brandeis has sponsored SciFest, though this was the first time alumni gave talks.
“Brandeis is special in the world of higher education not only because of the excellence one sees here in the research and teaching that goes on, but also due to the close connections that our undergraduate students are able to forge with doctoral students, postdocs and their professors,” President Ron Liebowitz said in opening remarks.
“These connections bring together students and researchers at multiple levels of scientific knowledge and sophistication which creates a truly unique environment in which our undergraduates early on learn the joys, challenges
In his talk to undergraduates, Ronny Drapkin ’90, director of the Ovarian Cancer Research Center at the University of Pennsylvania, spoke about the importance of spending large amounts of time in the lab doing research.
“There’s no substitute for working really hard,” he said. “The amount of time you spend on the bench is, I think, directly proportional to what you’re likely to” discover.
He also urged the aspiring scientists to remain alert to serendipitous breakthroughs.
“You have to be vigilant,” he said. “You don't know whether the next opportunity may be right under your nose.”
Robert Rottapel '79, the Amgen Chair for Cancer Research at Princess Margaret Cancer Centre in Toronto, said he chose to attend Brandeis because he wanted a school that had graduate programs in the sciences, “but was small because somehow I knew that I was the kind of personality that would flourish in a smaller, more intimate environment.”
“I hit the sweet spot by coming to this university,” he added.
Rottapel also spoke about the rich relationships he developed with faculty members. He remembered Henry F. Fischbach Professor of Chemistry Irving Epstein, then recently arrived at Brandeis, as a “young, brilliant professor” who taught classes where the average grade was 55 to 65. It was challenging, he said, but ultimately highly rewarding.
Rottapel said even music professors played a large role in his intellectual and professional development. Robert Koff, a teacher at Brandeis and founding member of the Juilliard String Quartet at New York’s Juilliard School, peppered his lectures with jokes.
Rottapel said this taught him, “No lesson is learned in the absence of humor.”
Leslie Meltzer ’03 described her career path from academia — she earned a
“Don't be afraid to step out of the box and try something new,” she said. “You won’t know what you like unless you try it.”
In the afternoon, the students presented the results of their summer research under the guidance of a Brandeis professor. Standing before posters, they explained their findings in roughly five minutes to attendees. The subjects ranged from high-energy physics to biomaterials and from quantum chemistry to fruit fly behavior.
Noah Somberg ’20 researched how Turing patterns form on biologically inspired growing systems.

Noah Somberg
Turing patterns explain how a simple set of chemical reactions can lead to the complex patterns found in nature — from zebra stripes to the distribution of vegetation across a landscape to the constellations of galaxies.
Somberg looked at a Turing pattern formed by the chlorine dioxide-iodine-malonic acid (CDIMA) reaction. He started with a symmetrical pattern, then used mathematical models to examine what shapes and arrangements resulted as the CDIMA reaction played out.
The resulting pattern changed when Somberg altered how fast the area of the Turing pattern grew. By understanding how the Turing pattern develops, Somberg says we may one day be able to harness the properties of nature to improve human well-being.
Henry F. Fischbach Professor of Chemistry Irving Epstein and associate professor of chemistry Milos Dolnik advised Somberg. Somberg’s research was funded by
Janelle Mabrey studied photosensitivity in a single-well chemical oscillator.

Janelle Mabrey
As part of a summer research program funded by the National Science Foundation, Brandeis' Materials Research Science and Engineering Center (MRSEC) hosts five students from Hampton University, a historically African-American university in Virginia. Mabrey begins her sophomore year at Hampton in the fall.
Mabrey looked at a strange and fascinating phenomenon called the Belousov–Zhabotinsky (BZ) reaction where the right combination of chemicals will oscillate between two states. A classic BZ reaction, for example, goes back and forth from red to blue.
In her experiments, Mabrey altered the intensity of the light shining during a BZ reaction, recording how it affected the compound’s oscillation. A low-intensity light slowed down the frequency of oscillations. She also explored how periods of light followed by periods of darkness changed the BZ reaction.
Certain biological functions — the firing of a neuron or electrical pulses passing through heart tissue — can be mimicked using the oscillating features of the BZ reaction. The aim of Mabrey and others’ research is to develop new materials that share these lifelike properties.
Professor of physics Seth Fraden advised Mabrey.
Jeff Liu ’20 and Michael Hsiao ’20, working with adviser Leslie Griffith, the Nancy Lurie Marks Professor of Neuroscience, researched how sleep is necessary, but not sufficient, for operant conditioning in Drosophila fruit flies.

Jeff Liu
Liu and Hsiao designed an ingenious device to study the relationship between sleep and learning in fruit flies. They built a self-enclosed tower with a camera positioned at the top. Toward the bottom were three motorized disks, arranged in a Y-shaped pattern. The disks contained sugar water in one half and water in the other.
When a fly entered the apparatus, the camera monitored which way it flew.
If the insect went left, the disk it was approaching spun so that the sugar was facing the animal. If the insect went right, the disk spun so that the fly encountered the water.
Flies are drawn to sugar (who isn’t?) so they learned that if they went left, they’d get a reward.
Liu and Hsiao observed that the flies that slept soon after they’d been placed into the chamber learned to head left. But flies that slept later, or not at all, never figured out that heading left led to sugar.
The findings are consistent with studies showing the importance of sleep for humans.
Liu and Hsiao’s research was funded by a Division of Science Summer Research Fellowship.
Categories: Alumni, Research, Science and Technology, Student Life





