Iu-Yam Chan
 Professor of Chemistry Emeritus
Professor of Chemistry Emeritus
Degrees
University of Chicago, PhD
Profile
Iu-Yam Chan is interested in laser spectroscopy and magnetic resonance under high pressure.
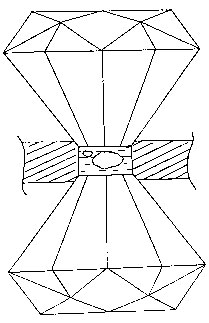 Fig.1 A diamond anvil cell
Fig.1 A diamond anvil cell
The derivative of free-energy vs. pressure is volume. Any change of state involving volume changes will have a pressure dependent equilibrium state, and a process having finite activation volume exhibits a pressure-dependent rate. Pressure ranks with temperature as a useful thermodynamic parameter. The experimental difficulty of pressure work has been alleviated in recent years through the availability of the diamond anvil cell (DAC). It exploits the superior mechanical strength of diamond. In a DAC, the sample is located in a small hole in a metal gasket. As the gasket is squeezed between two diamond anvils, extremely high pressure may be generated with a very compact apparatus, see Fig.1. Our DAC, reaching 100,000 atm, has a thermal mass of some 60 gram. Such a device may readily be cooled to liquid helium temperature. This opens up many scientific opportunities. A few years ago, we invented a technique to conduct magnetic resonance experiments in a DAC. This feat was achieved by finding a way to couple microwave energy into a small hole in a metal gasket. This breakthrough created a new dimension in magnetic resonance. We have made many interesting observations on the behavior of triplet state organic molecules under pressure.
A second area of our application is on pressure modification of chemical reaction rates. In particular, we focused on the rate of quantum tunneling reactions. An example is acridine-doped fluorene crystals. Upon excitation of the acridine to its lowest triplet state, it may abstract a hydrogen from the methylenic bridge of the fluorene molecule. This hydrogen tunneling rate increases exponentially for over two thousand fold with increasing pressure. In a recent exploit, the rate of high-spin to low-spin tunneling of a ferrous complex was enhanced nearly a billion fold for only 20 kbar, see Fig.2. These are very exciting results.
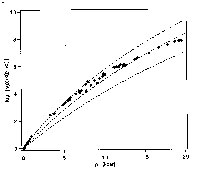 Fig. 2 Ration of the HS to LS tunneling rate to that at ambient pressure for a spin-crossover compound. The dash lines are theoretic curves for three different values of a parameter.
Fig. 2 Ration of the HS to LS tunneling rate to that at ambient pressure for a spin-crossover compound. The dash lines are theoretic curves for three different values of a parameter.
Selected Publications
"High Pressure Studies of the Acridine/fluorene Photoreaction: Vibration Assisted Tunneling", J. Chem. Phys. 103, 2959 (1995); with M.S. Dernis, C.M. Wong, B. Prass and D. Stehlik. Abstract
"Comparative Studies of Triplet Monocyclic Aromatic Diazines under Pressure", J. Chem. Phys. 104, 2476 (1996); with W. Wang. Abstract
"High Pressure Investigation of Absorption Spectra of J-aggregates", J. Chem. Phys. 104, 5359 (1996); with M. Lindrum. Abstract
"Pressure effects on the HS-LS Relaxation in [Zn1-xFex(6-mepy)3tren](PF6)2", J. Chem. Phys. 106, 3817 (1997); with W. Wang, S. Schenker and A. Hauser. Abstract