Didier Y.R. Stainier, PhD ‘84
Director & Scientific Member
Department of Developmental Genetics
Max Planck Institute for Heart and Lung Research
(Oct. 5, 2021)
Biological Robustness: Genetic Compensation and Transcriptional Adaption
It is a known phenomenon that when one sense is lost, such as the loss of sight, other senses are heightened in some way to compensate for the loss. Genetics research has identified a similar phenomenon. Dr. Stainier discussed his work exploring the mysteries of genetic compensation: in model organisms, turning off a particular gene (a knockout) can lead to other genes being increased, seemingly to compensate for the loss. This compensation was not seen when a gene was decreased (knockdown). Dr. Stainier and his lab refer to this as "transcriptional adaptation," and understanding this process could lead to greater understanding of genetic disorders and facilitate the design of new treatments.
The field of genetics has benefited from the genome engineering revolution: genes can be knocked out, knocked down or activated more easily than ever before. This range of genetic manipulations has also provided a range of outcomes, sometimes contradictory. But how much interesting biology hides within these discrepancies? Recent studies have shown that genetic compensation can be activated by some gene perturbations and not others, hinting that this phenomenon might skew our understanding of the genotype-phenotype relationship. In addition, the relatively frequent lack of phenotype in organisms from yeasts to humans when affected by a severe mutation is a paradox that has puzzled geneticists for decades.
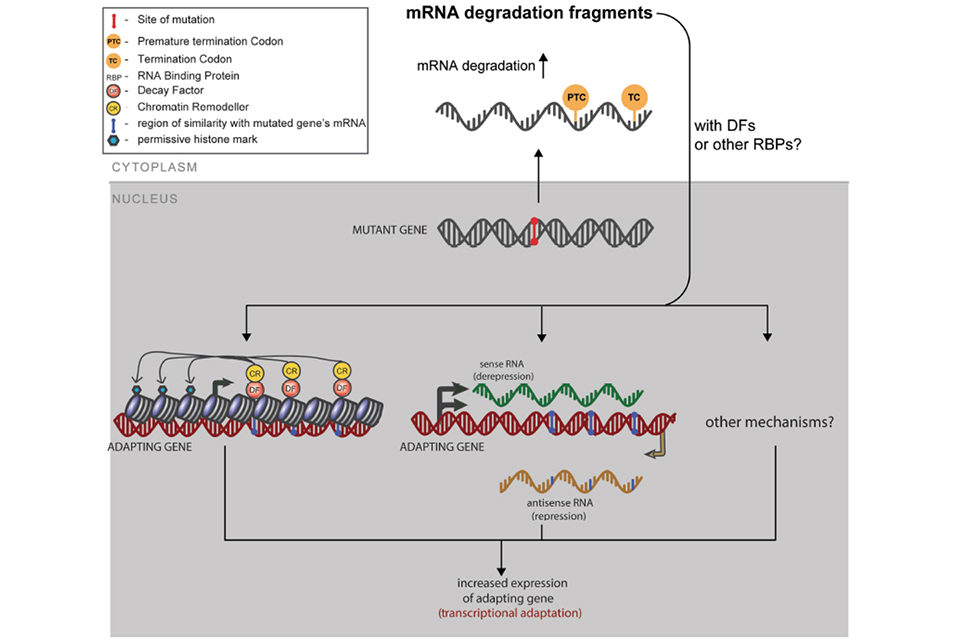
I will start by summarizing our work on trying to understand the surprising discrepancies observed between gene knockdown (e.g., antisense) and knockout (i.e., mutations) induced phenotypes. Working initially with zebrafish, my colleagues and I showed that knockout but not knockdown animals display genetic compensation by upregulating the expression of genes that can compensate for the loss of the mutated gene. We also provided evidence that this transcriptional upregulation, which we named "transcriptional adaptation," was independent of the loss of protein function.
We subsequently investigated the mechanisms underlying transcriptional adaptation in zebrafish embryos, mouse cell lines, and the worm C. elegans, and found that mutant mRNA degradation plays a critical role in activating this process. Additional data identified a role for small RNA biogenesis, and revealed potential mechanisms by which mRNA degradation fragments could activate the expression of related genes including by remodeling chromatin and/or inhibiting the function of antisense RNAs. Further evidence indicates that transcriptional adaptation is also at play in humans such that patients displaying mutant mRNA degradation often exhibit a milder phenotype than those who do not display mutant mRNA degradation. Thus, this newly identified phenomenon is very likely to have broad ranging implications not only in terms of our understanding of genetic and phenotypic robustness, but also possibly in designing more effective treatments for various genetic disorders.