Jeffrey Hall
 Professor Emeritus of Biology
Professor Emeritus of Biology
Jeffrey Hall, along with Michael Rosbash (Brandeis University), and Michael Young (Rockefeller University) received the Nobel Prize in Physiology or Medicine in 2017.
Research Description
Hall and his colleagues investigated the function of the nervous system in Drosophila. Many of their approaches involved genetic studies of behavior, augmented by molecular manipulations of genes defined by certain behavioral mutations.
These investigations were in two areas: molecular neurogenetics of courtship and molecular neurogenetics of biological rhythms.
Molecular Neurogenetics of Courtship
How is Drosophila's central nervous system programmed to control elements of the flies' reproductive behaviors? Our earlier studies that addressed this question involved identification of pertinent portions of the adult CNS, using "sex mosaics" and disruptions of these tissues, using mutants specifically defective in the metabolism of a centrally acting neurotransmitter, acetylcholine.
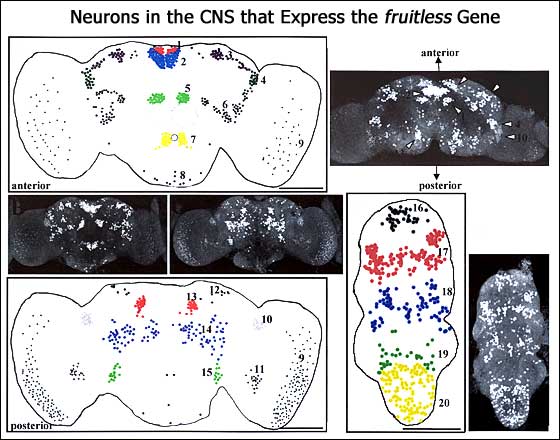
We then focused most of our attention on courtship mutants. One is defined by genetic changes at the fruitless (fru) locus, which cause males not to mate with females and anomalously to court other males. The genetics of fru are as complicated as the syndrome of behavioral abnormalities. We hoped to unravel some of these complexities by virtue identifying the fru at the molecular level. These and other neurobiological findings on the fru gene and its mutants have led to the demonstration that this factor acts within Drosophila's "sex-determination hierarchy" (SDH) and defines a new branch of it. In this context, fru, like certain other SDH genes, encodes a transcription factor. We discovered that production of serotonin (5HT), in certain of the many neurons that express male-specific forms of FRU protein, is regulated by the action of this gene. Moreover, the 5HT neuromodulator seems to regulate functions of male reproductive organs, which are dramatically perturbed in certain fru mutants.
Two other courtship mutants were isolated because of abnormal male "singing" behavior: cacophony (cac) and dissonance (diss) males generate aberrant sounds from the wing vibrations they direct at females. To approach an understanding of how these species-specific courtship songs are programmed into the fly, we carried out high resolution analyses of mutant vs. normal sounds. Genetic studies of the cac and diss loci implied complexities analogous to those associated with fru. For example, complementation analyses and molecular expression assessments indicate that both song genes are involved in the control of visually mediated responses made by the adult fly (as monitored behaviorally and physiologically). The cac gene encodes a voltage-regulated calcium channel (a1 subunit). This rationalizes at least the courtship-song defect in the cac mutant. The diss mutation is a variant of the nonA gene, which was defined originally by visual mutants and encodes an RNA-binding protein (NONA). We hypothesized that NONA influences post-transcriptional processing of the primary cac transcript. Indeed, there are several different isoforms of that mRNA, resulting from alternative splicing and RNA editing.
The rate at which Drosophila males generate the "tone pulse" components of their courtship song varies rhythmically. The cycle durations are short, ranging from about a half-minute to a minute-and-a-half, depending on the species. In one of these, D. melanogaster, circadian rhythm mutations at the period (per) locus cause song rhythm abnormalities that parallel the defects in "daily" rhythm phenotypes. These song findings provided entrées into our more extensive studies of biological rhythms.
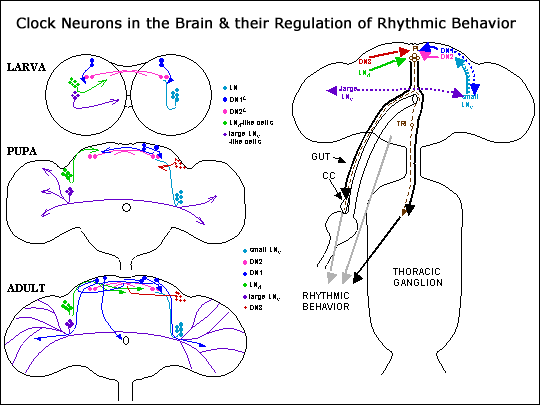
Molecular Neurogenetics of Biological Rhythms
We initiated molecular studies of circadian rhythms by analyzing the per gene, in collaboration with Michael Rosbash at Brandeis University. Through the 1990s, these investigations expanded to molecular and neurobiological experiments revolving round five additional rhythm-related genes in Drosophila, which were either cloned by others [timeless (tim)] or by us [Clock (Clk), cycle (cyc), and cryptochrome (cry), and pigment-dispersing factor (pdf)]. The various molecular-neuro-chronobiological investigations have involved:
- determination and manipulation (for bioasssays) of these genes' informational content
- in-vitro manipulations of clock genes or portions thereof--aimed in part at assessing the expression patterns of these genes at different stages of the life cycle and in a variety of neural and non-neural tissues; these studies included experimental identification of the neuronal pacemaker cells underlying the fly's behavioral circadian rhythms
- studies of environmental inputs to the circadian pacemaker, as mediated in part by cry function (which may be principally concerned with blue-light reception, but also seems connected to actual pacemaker operation), and probably by Clk and cyc as well (these are mainly clock factors, but the mutants also exhibit abnormal light-responsiveness in the context of daily rest-activity rhythms)
- manipulation of the pdf gene, which in Drosophila does not encode literally a pigment-dispersing factor--instead, an oligopeptide found only in the CNS; these experiments showed (to a first approximation) how PDF participates in output functions of the circadian clock, those underlying the flies' sleep-wake cycles
- comparison of the structure and function of clock genes in closely vs. distantly related Drosophila species; these data have revealed, for example that certain coding regions are highly conserved and others very diverged, among the different species; such findings led to interspecific transfer experiments, in which the effects of all or part of a donor species' per gene have been bioassayed in D. melanogaster hosts.
Selected Publications
- Hall J.C. (2000) Cryptochrome: sensory reception, transduction and clock functions subserving circadian systems. Curr. Opin. Neurobiol. 10: 456-466.
- Park J.H., Helfrich-Förster C., Lee G.H., Liu L., Rosbash M., and Hall J.C. (2000) Differential regulation of circadian pacemaker output by separate clock genes in Drosophila. Proc. Nat. Acad. Sci. U.S. 97: 3608-3613.
- Emery P., Stanewsky R., Hall J.C., and Rosbash M. (2000) A unique circadian-rhythm photoreceptor. Nature 404: 456-457.
- Emery P., Stanewsky R., Helfrich-Föster C., Emery-Le M., Hall J.C., and Rosbash M. (2000) Drosophila CRY is a deep brain circadian photoreceptor. Neuron 26:493-504.
- Kaneko M., and Hall J.C. (2000) Neuroanatomy of cells expressing clock genes in Drosophila: transgenic manipulation of the period and timeless genes to mark the perikarya of circadian pacemaker neurons and their projections. J. Comp. Neurol. 422: 66-94.
- Goodwin S.F., Taylor B.J. Villella A., Foss M., Ryner L.C., Baker B.S., and Hall J.C. (2000) Mutations in the fruitless gene of Drosophila melanogaster causing aberrant splicing or altered spatial distributions of fru's sex-specific expression patterns. Genetics 154: 725-745.
- Lee G., Foss M., Goodwin S.F., Carlo T., Taylor B.J., and Hall J.C. (2000) Spatial, temporal, and sexually dimorphic expression patterns of the fruitless gene in the Drosophila CNS. J. Neurobiol. 43: 404-426.
- Lee G., and Hall J.C. (2001) Abnormalities of male-specific FRU protein and serotonin expression in the central nervous system of fruitless mutants in Drosophila. J. Neurosci.21: 513-526.
- Lee G., Villella A., Taylor B.J., and Hall J.C. (2001) New reproductive anomalies in fruitless-mutant Drosophila males: extreme lengthening of mating durations and infertility correlated with defective serotonergic innervation of reproductive organs. J. Neurobiol. 47: 121-149.
- Baker B.S., Taylor B.J., and Hall J.C. (2001) Are complex behaviors specified by dedicated regulatory genes? Reasoning from Drosophila. Cell 105: 13-24.
- Helfrich-Forster C., Winter C., Hofbauer A., Hall J.C., and Stanewsky R. (2001) The circadian clock of Drosophila is blind after elimination of all known photoreceptors. Neuron. 30: 249-261.
- Krishnan B., Levine J.D., Lynch K.S., Dowse H.B., Funes P., Hall J.C., Hardin P.E., and Dryer S.E. (2001) A novel role for cryptochrome in a Drosophila circadian oscillator. Nature. 411: 313-317.
- Campesan S., Dubrova Y., Hall J.C., and Kyriacou C.P. (2001) The nonA gene in Drosophila conveys species-specific behavioral characteristics. Genetics 158: 1535-1543.
- Stempfl, T., Vogel M., Szabo G., Wülbeck C., Liu J., Hall J.C., and Stanewsky R. (2002) Identification of circadian-clock regulated enhancers and genes of Drosophila melanogaster by transposon mobilization using firefly luciferase as a reporter. Genetics 160: 571-593.
- Chan B., Villella A., Funes P., and Hall J.C. (2002) Courtship and other behaviors affected by a heat-sensitive, molecularly novel mutation in the cacophony calcium-channel gene of Drosophila. Genetics 162: 135-153.
- Levine J.D., Funes P., Dowse H.B., and Hall J.C. (2002) Resetting the circadian clock by social experience in Drosophila melanogaster. Science 298: 2010-2012.
- Hall J.C. (2003) Genetics and molecular biology of rhythms in Drosophila and other insects. Adv. Genet. 48: 1-286.
- Peng Y., Stoleru D., Levine J.D., Hall J.C., and Rosbash M. (2003) Drosophila free-running rhythms require intercellular communication. Pub. Lib. Sci. Biol. 1: 32-40.
- Hall J.C. (2003) A neurogeneticist's manifesto. J. Neurogenet. 17:1-90.
- Veleri S., Brandes C., Helfrich-Förster C., Hall J.C., and Stanewsky R. (2003) A self-sustaining, light-entrainable circadian oscillator in the brain of Drosophila. Curr. Biol. 13: 1758-1767.
- Shafer O., Levine J.D., Truman J.W., and Hall J.C. (2004) Flies by night: effects of changing day length on Drosophila's circadian clock. Curr. Biol. 14: 424-432.
- Choi Y., Lee G., Hall J.C., and Park J.H. (2005) Cloning and expression analysis of corazonin-encoding genes in Drosophila species and functional insights into corazonin-expressing neurons. J. Comp. Neurol. 482: 372-385.
- Hall J.C. (2005) Systems approaches to biological rhythms in Drosophila. Methods Enzymol. 393: 61-185.
- Manoli D.S., Foss M., Villella A., Taylor B.J., Hall J.C., and Baker B.S. (2005) Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature 436: 395-400.
- Villella A., Ferri S.L., Krystal J.D., and Hall J.C. (2005) Functional analysis of fruitless gene expression by transgenic manipulations of Drosophila courtship. Proc. Nat. Acad. Sci. U.S. 102: 16550-16557.
- Kadener S, Villella A, Kula E, Palm K, Pyza E, Botas J, Hall JC, Rosbash M. (2006) Neurotoxic protein expression reveals connections between the circadian clock and mating behavior in Drosophila. Proc Natl Acad Sci U S A. 2006 Sep 5;103:13537-42.
- Villella A, Peyre JB, Aigaki T, Hall JC. (2006) Defective transfer of seminal-fluid materials during matings of semi-fertile fruitless mutants in Drosophila. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006 Dec;192:1253-69.