Shantanu Jadhav
 Professor of Psychology
Professor of Psychology
Research Description
Learning, Memory and Decision Making in the Mammalian Brain
The brain has a remarkable capacity to learn and to use past experience to guide our daily behavior. Multiple brain regions coordinate activity to form representations of the external world, learn new experiences, store and retrieve memories, and make decisions. We are interested in understanding the neural basis of these cognitive abilities by studying processing at the cellular and network level in the neuronal circuits of the rodent brain.
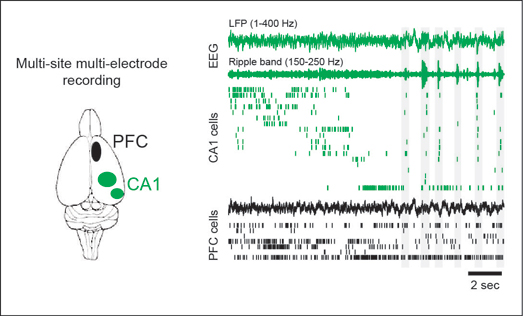
We are currently investigating how two critical brain regions, the hippocampus and the prefrontal cortex, interact and communicate with each other to support learning and memory, working memory and memory-guided decision making. The hippocampus is known to be critical for episodic memories, and the prefrontal cortex is involved in executive control, working memory and decision making. Communication between the prefrontal executive system and the hippocampal memory system is key for learning, remembering, planning, prediction and memory-guided decision making. However, the nature of communication between these two regions, and the neural mechanisms, fine timescale structure, and causal contributions of this pivotal interaction remain largely unknown. How does ensemble neural activity in these structures evolve during learning, what mechanisms underlie the organization and transmission of activity in ensembles of neurons across these structures, what role do network activity patterns seen in these structures such as theta, gamma and sharp-wave ripple oscillations play in local and inter-regional processing, and how do these patterns of activity relate to memory and decision making during ongoing behavior?
We address these questions using a combination of techniques, including behavior, large scale multielectrode recordings in awake behaving animals, real time detection and perturbation of neural activity patterns, targeted optogenetic interventions, and computational analysis. We have shown that hippocampal replay during awake sharp-wave ripples (SWRs) is critical for spatial memory, and SWRs are associated with coordinated reactivation of hippocampal-prefrontal neurons during memory-guided decision making. This approach thus allows us to characterize the neurophysiological basis of prefrontal-hippocampal interactions, and also to provide causal evidence linking specific forms of neural activity to behavior and cognition. This will provide crucial insight into several neurological and neuropsychiatric disorders involving these two regions, such as dementia, Alzheimers, depression and schizophrenia.
Selected Publications
-
Ding M, Tomsick PA, Young RA, Jadhav SP (2025), “Ventral tegmental area dopamine neural activity switches simultaneously with rule representations in the prefrontal cortex and hippocampus”, Journal of Neuroscience, 45(37):e1670242025. Cover Article, Special Issue: Computational Properties of Prefrontal Cortex.
- Young RA, Shin JD, Guo Z, Jadhav SP (2025), “Hippocampal-prefrontal communication subspaces align with behavioral and network patterns in a spatial memory task”, eNeuro, ENEURO.0336-24.2025.
- Porter BS, Olson JM, Leppla CA, Duvelle E, Bladon JH, van der Meer M, Jadhav SP (2025), “Adapt-A-Maze: An Open Source Adaptable and Automated Rodent Behavior Maze System”, eNeuro, ENEURO.0138-25.2025.
- Shin JD, Jadhav SP (2024), “Prefrontal cortical ripples mediate top-down suppression of hippocampal reactivation during sleep memory consolidation”, Current Biology, 34:2801-2811.e9.
- Breffle J, Germaine H, Shin JD, Jadhav SP*, Miller P* (2024), “Intrinsic dynamics of randomly clustered networks generate place fields and preplay of novel environments”, eLife, 2024 Oct 18:13:RP93981.
- Tang W*, Shin JD*, Jadhav SP (2023), “Geometric transformation of cognitive maps for generalization across hippocampal-prefrontal circuits”, Cell Reports, 42:112246.
- Symanski CA, Bladon JH, Kullberg ET, Miller P, Jadhav SP (2022), “Rhythmic coordination and ensemble dynamics in the hippocampal-prefrontal network during odor-place associative memory and decision making”, eLife, 11: e79545.
- Tang W, Jadhav SP (2022), “Multiple-timescale representations of space: linking memory to navigation”, Annual Review of Neuroscience, 45:1-21.
- Tang W*, Shin JD*, Jadhav SP (2021), “Multiple time-scales of decision making in the hippocampus and prefrontal cortex”, eLife, 10:e66227.
- Herzog LE, Katz DB, Jadhav SP (2020), “Refinement and reactivation of a taste-responsive hippocampal network”, Current Biology, 30:1306-1311.
- Zielinski MC, Tang W, Jadhav SP (2020), “The role of replay and theta sequences in mediating hippocampal-prefrontal interactions for memory and cognition”, Hippocampus, 30(1):60-72.
- Shin JD*, Tang W*, Jadhav SP (2019), “Dynamics of awake hippocampal-prefrontal replay for spatial learning and memory-guided decision making”, Neuron, 104(6):1110-1125.
- Zielinski MC, Shin JD, Jadhav SP (2019), “Coherent coding of spatial position mediated by theta oscillations in the hippocampus and prefrontal cortex”, Journal of Neuroscience, 39(23):4550-4565.
- Herzog LE, Pascual LM, Scott SJ, Mathieson ER, Katz DB, Jadhav SP (2019), “Interaction of taste and place coding in the hippocampus”, Journal of Neuroscience, 39(16):3057-3069.
- Tang W, Jadhav SP (2019), “Sharp-wave ripples as a signature of hippocampal-prefrontal reactivation for memory during sleep and waking states”, Neurobiology of Learning and Memory, 160:11-20.
- Maharjan DM, Dai Y, Glantz EH, Jadhav SP (2018), “Disruption of dorsal hippocampal-prefrontal interactions using chemogenetic inactivation impairs spatial learning”, Neurobiology of Learning and Memory, 155(1):351-360
- Tang W, Shin JD, Frank LM, Jadhav SP (2017), “Hippocampal-prefrontal reactivation during learning is stronger in awake compared with sleep states”, Journal of Neuroscience, 37(49): 11789-11805.
- Shin JD and Jadhav SP (2016), “Multiple modes of hippocampal-prefrontal interactions in memory-guided behavior”, Current Opinion in Neurobiology, 40:161-169.
-
Jadhav SP*, Rothschild G*, Roumis DK and Frank LM (2016). "Coordinated Excitation and Inhibition of Prefrontal Ensembles during Awake Hippocampal Sharp-Wave Ripple Events." Neuron 90(1): 113-127.
-
Jadhav SP, Kemere C, German PW, Frank LM (2012), “Awake hippocampal sharp-wave ripples support spatial memory”, Science. 336(6087): 1454-1458.
-
Carr MF*, Jadhav SP*, Frank LM (2011), “Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval”, Nature Neuroscience. 14:147-153. (*Equal author contribution, Peer-reviewed review article).
-
Jadhav SP, Feldman DE (2010), “Texture coding in the whisker system”, Current Opinion in Neurobiology. 20: 1-6. (Peer-reviewed review article).
- Jadhav SP, Wolfe J, Feldman DE (2009), “Sparse temporal coding of elementary tactile features during active whisker sensation”, Nature Neuroscience,12(6):792-800.
- Gabernet L, Jadhav SP, Feldman DE, Carandini M, Scanziani M (2005), “Somatosensory integration controlled by dynamic thalamocortical feed-forward inhibition”, Neuron, 48:315-327.