Daniel Oprian
 Louis and Bessie Rosenfield Professor of Biochemistry
Louis and Bessie Rosenfield Professor of Biochemistry
Research Description
Structure-function studies of visual pigments and other cell surface receptors
Our laboratory is interested in the structure and function of G protein-coupled receptors with particular focus on the subgroup of receptors known as visual pigments. The pigments are major components of rod and cone photoreceptor cells and form the basis of phototransduction in the vertebrate retina. As is typical of G protein-coupled receptors, the visual pigments are integral membrane proteins composed of seven transmembrane helical segments. However, what is atypical is that each pigment is bound covalently to a small molecule ligand, 11-cis-retinal, which is a chromophore for the absorption of light.
Spectral Tuning
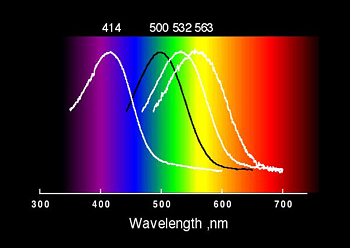
There are four visual pigments in the human retina: rhodopsin, the pigment of rod photoreceptor cells, and the blue, green and red color vision pigments of cone photoreceptor cells. These four pigments have absorption spectra which span (actually define) the visible region of the electromagnetic spectrum. And yet, the small molecule chromophore responsible for absorption of light is the same in each pigment. One of the goals of our research is to understand the underlying mechanism by which the spectrum of each pigment is “tuned” by interactions of the retinal chromophore with amino acid side chains in the active site of the proteins.
Mechanism of Retinal Disease
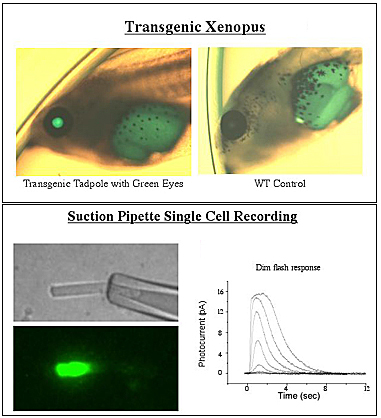 A large number of mutations in rhodopsin are known to cause inherited diseases of the retina. We are interested in a small group of these which result in constitutive activation of rhodopsin - that is, these mutations cause the protein to activate the G protein transducin in the absence of light. The activating mutations are known to cause two different diseases: a devastating degenerative disease of the retina known as retinitis pigmentosa and, in comparison, a relatively benign disease known as congenital stationary night blindness. Our goal is to elucidate the underlying molecular mechanisms responsible for pathophysiology in these diseases. To do this, we employ a broad array of techniques ranging from classical biochemistry and mutagenesis for in vitro characterization of the mutant proteins to the development of transgenic animals combined with single-cell electrophysiology to test models of disease under in vivo conditions.
A large number of mutations in rhodopsin are known to cause inherited diseases of the retina. We are interested in a small group of these which result in constitutive activation of rhodopsin - that is, these mutations cause the protein to activate the G protein transducin in the absence of light. The activating mutations are known to cause two different diseases: a devastating degenerative disease of the retina known as retinitis pigmentosa and, in comparison, a relatively benign disease known as congenital stationary night blindness. Our goal is to elucidate the underlying molecular mechanisms responsible for pathophysiology in these diseases. To do this, we employ a broad array of techniques ranging from classical biochemistry and mutagenesis for in vitro characterization of the mutant proteins to the development of transgenic animals combined with single-cell electrophysiology to test models of disease under in vivo conditions.
Structure of the Active State
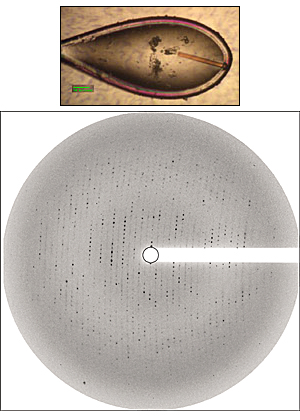
Rhodopsin is the only G protein-coupled receptor for which a structure has been determined by x-ray crystallography. While the structure of rhodopsin has had an enormous impact on our understanding of the protein it is clear that we are at the very beginning of these structural studies and major questions remain. In particular, we still do not have a structure for the active conformation of the protein – the published rhodopsin structure is for the inactive- or dark-state. Our laboratory is currently undertaking a major effort to determine the x-ray crystal structure of the active state of rhodopsin. In addition, we are undertaking efforts to determine the crystal structure of other G protein-coupled receptors.
Selected Publications
- Matos JO, Kumar RP, Ma AC, Patterson M, Krauss IJ, Oprian DD. Mechanism Underlying Anti-Markovnikov Addition in the Reaction of Pentalenene Synthase. Biochemistry. 2020 Sep 8;59(35):3271-3283. doi: 10.1021/acs.biochem.0c00518.
- Ozdeslik RN, Olinski LE, Trieu MM, Oprian DD, Oancea E. Human nonvisual opsin 3 regulates pigmentation of epidermal melanocytes through functional interaction with melanocortin 1 receptor. Proc Natl Acad Sci U S A. 2019 Jun 4;116(23):11508-11517. doi: 10.1073/pnas.1902825116.
- Morehouse BR, Kumar RP, Matos JO, Yu Q, Bannister A, Malik K, Temme JS, Krauss IJ, Oprian DD. Direct Evidence of an Enzyme-Generated LPP Intermediate in (+)-Limonene Synthase Using a Fluorinated GPP Substrate Analog. ACS Chem Biol. 2019 Sep 20;14(9):2035-2043. doi: 10.1021/acschembio.9b00514.
- Kumar RP, Morehouse BR, Matos JO, Malik K, Lin H, Krauss IJ and Oprian DD (2017). "Structural Characterization of Early Michaelis Complexes in the Reaction Catalyzed by (+)-Limonene Synthase from Citrus sinensis Using Fluorinated Substrate Analogues." Biochemistry. 56 (12), pp 1706–1715.
- Morehouse BR, Kumar RP, Matos JO, Olsen SN, Entova S and Oprian DD (2017). "Functional and Structural Characterization of a (+)-Limonene Synthase from Citrus sinensis."Biochemistry. 56 (12), pp 1706–1715.
- Chakrabarti KS, Agafonov RV, Pontiggia F, Otten R, Higgins MK, Schertler GF, Oprian DD and Kern D (2016). "Conformational Selection in a Protein-Protein Interaction Revealed by Dynamic Pathway Analysis." Cell Rep 14(1): 32-42.
- Devine EL, Theobald DL and Oprian DD (2016). "Relocating the Active-Site Lysine in Rhodopsin: 2. Evolutionary Intermediates." Biochemistry 55(34): 4864-4870
- Kumar RP, Ranaghan MJ, Ganjei AY and Oprian DD (2015). "Crystal Structure of Recoverin with Calcium Ions Bound to Both Functional EF Hands." Biochemistry54(49): 7222-7228.
- D'Antona AM, Xie G, Sligar SG and Oprian DD (2014). "Assembly of an activated rhodopsin-transducin complex in nanoscale lipid bilayers." Biochemistry 53(1): 127-134.
- Devine EL, Oprian DD and Theobald DL (2013). "Relocating the active-site lysine in rhodopsin and implications for evolution of retinylidene proteins." Proc Natl Acad Sci U S A 110(33): 13351-13355.
- Ranaghan MJ, Kumar RP, Chakrabarti KS, Buosi V, Kern D and Oprian DD (2013). "A highly conserved cysteine of neuronal calcium-sensing proteins controls cooperative binding of Ca2+ to recoverin." J Biol Chem 288(50): 36160-36167.
- Deupi X, Edwards P, Singhal A, Nickle B, Oprian DD, Schertler G and Standfuss J (2012). "Stabilized G protein binding site in the structure of constitutively active metarhodopsin-II." Proc Natl Acad Sci U S A 109(1): 119-124.
- Frederiksen R, Boyer NP, Nickle B, Chakrabarti KS, Koutalos Y, Crouch RK, Oprian D and Cornwall MC (2012). "Low aqueous solubility of 11-cis-retinal limits the rate of pigment formation and dark adaptation in salamander rods." Journal of General Physiology 139(6): 493-505.
- Standfuss J, Edwards PC, D'Antona A, Fransen M, Xie G, Oprian DD and Schertler GF (2011). "The structural basis of agonist-induced activation in constitutively active rhodopsin." Nature 471(7340): 656-660.
- Xie G, D'Antona AM, Edwards PC, Fransen M, Standfuss J, Schertler GF and Oprian DD (2011). "Preparation of an activated rhodopsin/transducin complex using a constitutively active mutant of rhodopsin." Biochemistry 50(47): 10399-10407.
- Standfuss J, Xie G, Edwards PC, Burghammer M, Oprian DD, Schertler GF (2007). "Crystal structure of a thermally stable rhodopsin mutant." J Mol Biol. 2007 Oct 5;372(5):1179-88.
- Bayburt TH, Leitz AJ, Xie G, Oprian DD, Sligar SG (2007). " Transducin activation by nanoscale lipid bilayers containing one and two rhodopsins." J Biol Chem. 2007 May 18;282(20):14875-81.
- Chen J, Shi G, Concepcion FA, Xie G, Oprian D, Chen J (2006). "Stable rhodopsin/arrestin complex leads to retinal degeneration in a transgenic mouse model of autosomal dominant retinitis pigmentosa." J Neurosci. 2006 Nov 15;26(46):11929-37.
- Higgins MK, Oprian DD, Schertler GF (2006). "Recoverin binds exclusively to an amphipathic peptide at the N terminus of rhodopsin kinase, inhibiting rhodopsin phosphorylation without affecting catalytic activity of the kinase." J Biol Chem. 2006 Jul 14;281 (28):19426-32.
- Tam BM, Xie G, Oprian DD, Moritz OL (2006). "Mislocalized rhodopsin does not require activation to cause retinal degeneration and neurite outgrowth in Xenopus laevis." J Neurosci. 2006 Jan 4;26(1):203-9.
- Kono M, Crouch RK, Oprian DD (2005). "A dark and constitutively active mutant of the tiger salamander UV pigment." Biochemistry. 44:799-804.
- Kim JM, Altenbach C, Kono M, Oprian DD, Hubbell WL, Khorana HG (2004). "Structural origins of constitutive activation in rhodopsin: Role of the K296/E113 salt bridge." Proc Natl Acad Sci USA. 101(34):12508-13.
- Das J, Crouch RK, Ma JX, Oprian DD, Kono M (2004). "Role of the 9-methyl group of retinal in cone visual pigments." Biochemistry. 43(18):5532-8.
- Jin S, Cornwall MC, Oprian DD (2003). "Opsin activation as a cause of congenital night blindness." Nat Neurosci. 6:731-5.
- Jin S, McKee TD, Oprian DD (2003). "An improved rhodopsin/EGFP fusion protein for use in the generation of transgenic Xenopus laevis." FEBS Lett. 542(1-3):142-6.
- Oprian DD (2003). "Phototaxis, chemotaxis and the missing link." Trends Biochem Sci. 28:167-9.
- Gross AK, Rao VR, Oprian DD (2003). "Characterization of rhodopsin congenital night blindness mutant T94I." Biochemistry. 42:2009-15.
- Gross AK, Xie G, Oprian DD (2003). "Slow binding of retinal to rhodopsin mutants G90D and T94D." Biochemistry. 42:2002-8.
- Xie G, Gross AK, Oprian DD (2003). "An opsin mutant with increased thermal stability." Biochemistry. 42:1995-2001.