SARS-CoV-2 Testing: Demystifying the Terminology
As the United States enters its fourth month of response to the COVID-19 crisis, many are pointing to the testing of people potentially infected with the disease as the key to effective mitigation of the public health threat. Much confusion exists about testing for SARS-CoV-2, the virus that causes the disease COVID-19. For instance, you may have heard of PCR as a method, but what is PCR, and how does it differ from antibody tests, another much-touted testing modality? We spoke to Director of Licensing and Strategic Alliances Rajnish Kaushik, a virologist by training, as well as Prof. Emeritus Lawrence Wangh, a globally recognized expert on PCR, to understand more about the types of tests used to determine whether someone has been infected with the virus that causes COVID-19, or, indeed, many other infectious agents. Please note that this summary of commonly-used scientific processes is not intended as medical or public health advice. It’s meant to be a handy deeper explanation of scientific terms you may be hearing about in the news, to help you better understand news coverage.
There are two types of tests discussed in the media: tests to determine whether someone is currently infected with a virus, and those meant to identify whether someone had the virus in the past. For the first kind of test, there are two options: PCR and isothermal amplification technologies.
The Science of Rapid Testing
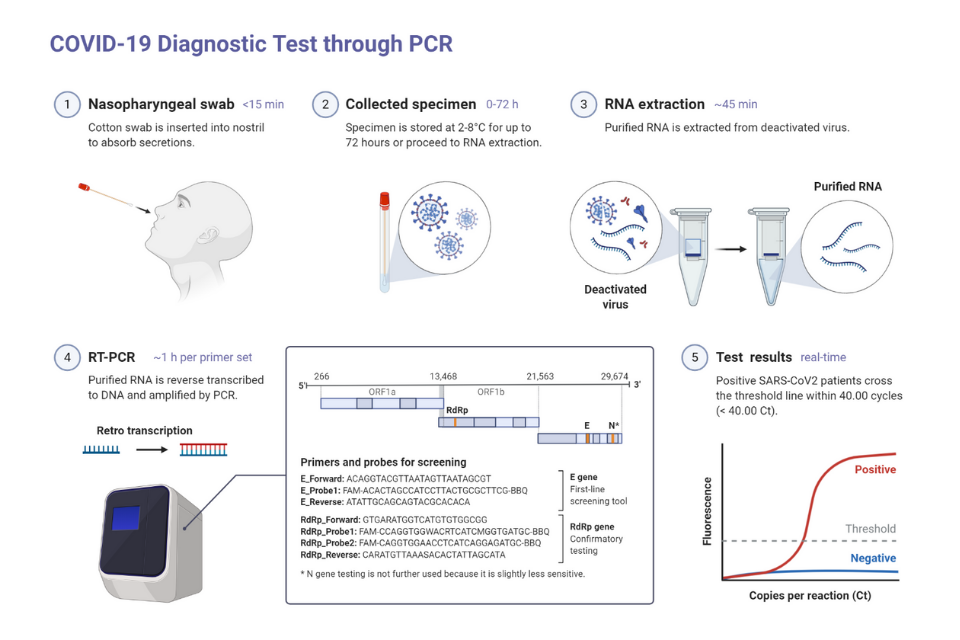
PCR stands for polymerase chain reaction, which is a method for rapidly making copies of a strand of DNA. It’s used in a variety of scientific applications, including genetic sequencing for detecting genetic disorders, or forensic tests popularly known as genetic fingerprinting. It’s also useful in identifying the germs that may infect us. Many viruses, including SARS-CoV-2, contain an RNA genome, so they need to first be reverse-transcribed into DNA. Then, the DNA is examined by the PCR method using virus-specific primers to determine whether the sample has that specific virus. This is quantitative reverse transcription PCR (RT-qPCR), which means that this extra step was needed before PCR. With PCR, scientists compare a sample taken from a suspected patient, say, of saliva, that would contain the virus if the person is infected. They then extract the genetic material, RNA, transcribe it into DNA, then use PCR to amplify that DNA with primers specific to the genetic sequence of SARS-CoV-2. If the DNA is amplified then the machine detects the signal as positive and the patient is likely infected with the virus.
But what if a person comes into a healthcare facility showing similar symptoms, and the PCR test shows that they are not infected with the specific virus for which they were tested? They may be sent home without an answer, or more tests are needed. In a public health crisis where managing community spread of a specific virus is the main goal, PCR is a rapid, efficient way to determine who carries the one virus of concern. However, in a medical setting, and in many public health contexts, simply knowing what a patient doesn’t have is only a partial answer. Identifying the infectious agent exactly, rather than ruling out one single infectious agent, is important in many settings. Also, PCR detection can be time-consuming, expensive, needs heavy equipment, and may not be done at the testing site.
Enter isothermal nucleic acid amplification technology. It doesn’t require complex PCR machines or multiple cycles of heating and cooling the reaction mixture. While this process also requires reverse transcription of the genetic material of RNA viruses, it then uses a DNA polymerase to rapidly generate multiple copies of virus-specific DNA using virus-specific primers. The key advantage of this technology is the rapidity with which the diagnosis can be made. Also, it doesn’t require heavy equipment and can easily be used as a point of care device at the testing site. Both PCR and isothermal nucleic acid amplification technologies can be multiplexed, in other words, set up so that multiple tests run concurrently, to diagnose more than one pathogen from the same sample though these amplification assays. As a point of care device, isothermal nucleic acid amplification testing equipment delivers the result much faster. Multiplex diagnostic assays can help teams do more than rule-in or out a single infectious agent, instead giving them a faster diagnosis when symptoms, such as a cough, are common to many different infections.
What Exactly Are Antibody Tests?
Finally, there are antibody tests. When people are infected with a virus, their immune systems produce a range of molecules to fight the infection. This includes antibodies of the type immunoglobulin M (IgM) and G (IgG). IgM is the first antibody launched in the immune response from the host after the infection followed by more long-lasting IgG antibodies. IgG forms to help the body identify an infectious agent should it encounter it again. If the virus is ever again present in the patient, the IgG antibodies will bind to it and signal the body to deploy the immune response against that infectious agent at much shorter notice. It’s this binding action that is tested for in antibody tests. Since it takes time for the body to create, it’s often, though not always, a sign of past infection. While the body produces many antibodies in a range of contexts, when you hear of “antibody tests” in the context of the public health crisis, the relevant ones are those that test for the presence of IgG or IgM in the bloodstream. This would most likely indicate a past infection.
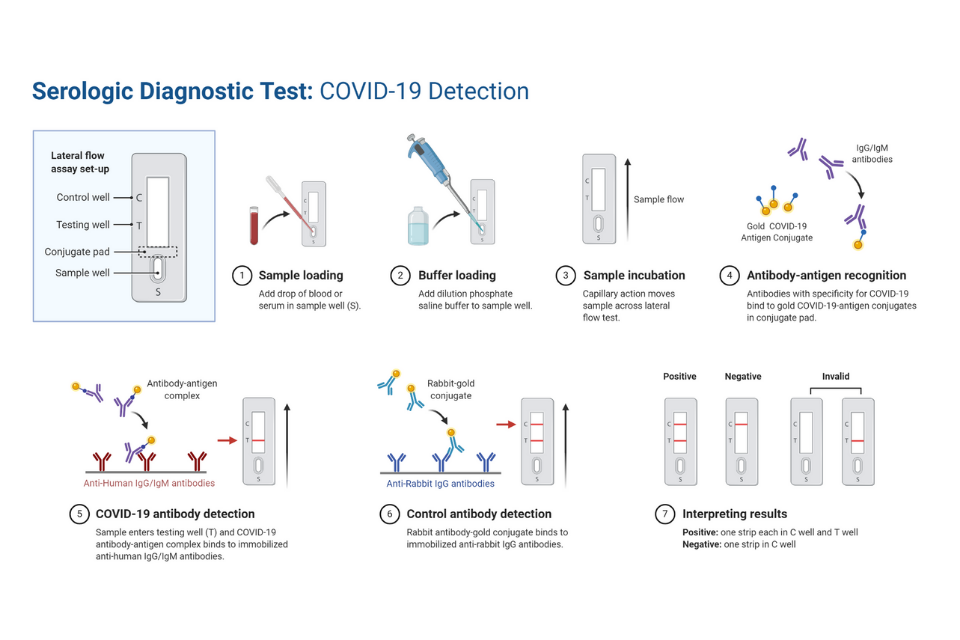
Much like tests for active infection, tests for antibodies come in different types. Lateral flow assays (LFA) take a small blood or saliva sample from a patient and flow it through a strip that contains virus particles. If the blood contains IgG/IgM specific to that virus, those antibodies will attach to the viral proteins. If there are enough antibodies in the blood sample, they will adhere to the viral proteins in large enough numbers to show as lines on the strip, similar to a pregnancy test.
Antibody tests can also involve enzyme-linked immunosorbent assays (ELISA), which on the other hand, use a plate coated with proteins from a virus, as well as a substance that will turn fluorescent when it’s around molecules that have bound together. A blood or saliva sample from a patient is placed on the plate. If IgG/IgM specific to the virus is present in the sample, those antibodies will bind to the viral protein. The substance that becomes fluorescent when in the presence of bound molecules will start to glow, verifying the presence of the specific antibodies. Like isothermal amplification, LFA provides rapid on-site detection as compared to ELISA, which takes more time and can only be done in clinical labs.
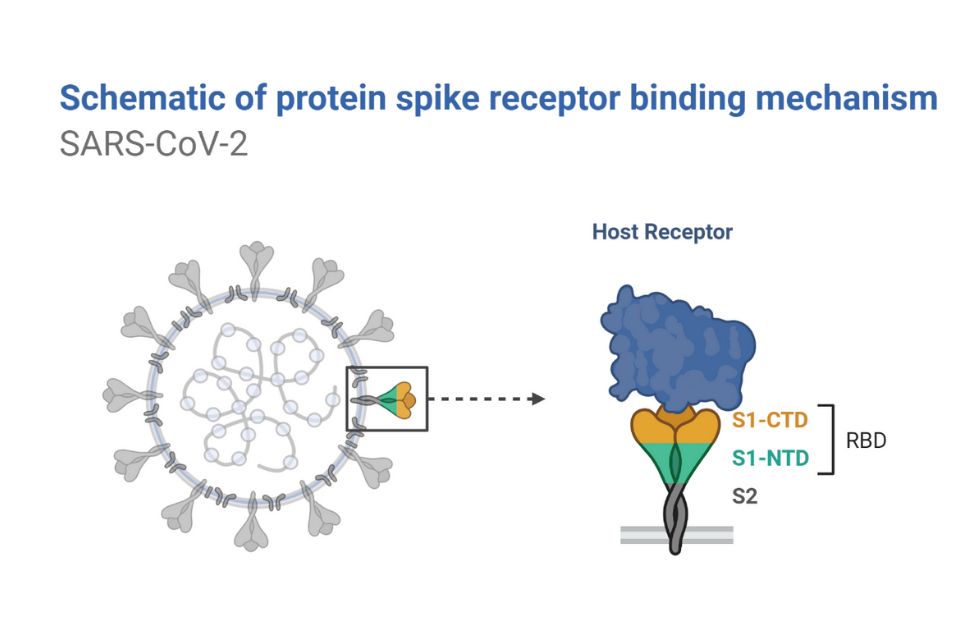
Antibody tests vary greatly in accuracy. This is for several reasons. One is human error - a faint glow or band may seem a stronger indicator of the presence of antibodies to some testers than others. Another is the type of viral protein used to make the test. Many viruses have a couple of proteins on their outer shells, including the proteins making up the shell itself, as well as the spike, a protruding structure that plays a role in helping the virus attach to its victims’ cells. Sometimes, these proteins can be similar among related viruses, meaning that a protein making up the shell of SARS-CoV-2 may be similar to the proteins encapsulating other coronaviruses, such as those which cause the common cold. If they are similar enough, antibodies for another virus may attach to proteins from SARS-CoV-2, giving false-positive results for SARS-CoV-2. Some tests may even use an incorrect protein, one from a different but similar virus. The spike proteins on SARS-CoV-2 are more distinctive than its shell proteins, so more recent tests use those spike proteins to get more accurate results, ensuring that they are identifying antibodies that truly target the novel coronavirus.
The Bottom Line
Both nucleic acid and antibody detection have their own pros and cons. While nucleic acid-based detection can be more accurate, it can only recognize the currently infected patients, whereas antibody tests can detect the infection even in people who were infected previously but now cleared of that virus. Accurate antibody tests can provide a more realistic number in terms of the percent of total infected individuals in the population who may also be immune to the virus, though more research is being done to confirm this.
Again, this is not medical advice. Please contact your healthcare practitioner if you have any questions or concerns about your own health. As questions about deploying tests become a concern for many around the world, knowing the science behind virus testing can shed light on the many logistical challenges communities face in testing members. This year, more than ever, understanding scientific terminology can help you be a more informed consumer of news reports and updates.